
State the correct IUPAC name of the following hydrocarbons. If you wish, you may return to the test and attempt to improve your score. Edpuzzle: IUPAC Nomenclature of Alkenes and Alkynes Worksheet Answer Key.Organic Chemistry Exercises. Compounds of carbon and hydrogen are called hydrocarbons.Organic Chemistry Generalized Organic Chemistry Naming Procedure Grouped into. The most widely used system of nomenclature is that formulated by the International Union for Pure and Applied Chemistry. Because the number of known organic structures runs to many millions, and the number of possible structures is infinite, a sophisticated nomenclature system that is capable of naming structures unambiguously is needed.
...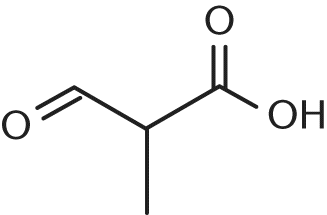
Alkoxy + alkane = Alkoxyalkane FormulaGeneral Formula: C nH 2n+1 COOH or R-COOHIUPAC names: Replace terminal e from the name of the corresponding alkane by the suffix oic acidI.e. FormulaThe general formula of trihaloalkanes is C nH 2n-1X 3 while that of tetrahaloalkanes is C nH 2n-2X 4 where n=1,2,3,4….The position of the halogen atoms on the carbon chain be indicated by Arabic numerals.Alcohols are classified as monohydric ,dihydric, trihydric and polyhydric according as their molecule contains 1,2,3 and many hydroxyl groups.Presence of two or more hydroxyl groups on the same carbon atom makes the molecule unstable, therefore, in di, tri and polyhydric alcohol , each hydroxyl group is present on a different carbon atom.General formula: C n H 2n+1 OH (where n=1,2,3…) or R-OHCommon names: Add the word alcohol to the name of alkyl group i.e.IUPAC names: Replace the terminal e from the name of corresponding alkane by the suffix ol.Because of their sweet taste ,dihydric alcohols are called glycols.Depending upon the relative position of the two hydroxyl groups, they are further classified as α, β, γ …ω glycols.Α-glycols are named by adding the word glycol to the common name of the alkene from which they have been prepared by direct hydroxylation.Β, γ ,ω glycols are named as the corresponding polymethylene glycols.IUPAC name: Add the suffix diol to the name of the alkane containing the same number of carbon atoms as the diol.IUPAC name: Add the suffix triol to the name of the alkane containing the same number of carbon atoms as the triol.The position of the hydroxyl group is indicated by arabic numerals.General Formula: R-O-R’ where R and R’ are same or different alkyl group.If R=R’ ,ethers are called simple ethers and if R≠R’ then ethers are called mixed ethers.Common names: In mixed ethers, add the word ether to the name of the alkyl groups arranged in alphabetical order.In simple ethers , the numerical prefix di is added to the name of the alkyl group followed by the word ether.IUPAC name: In the IUPAC system, ethers are called alkoxyalkanes.The smaller alkyl group forms a part of the alkoxy group while the bigger alkyl group forms a part of the alkane.The name of the ethers are then derived by adding the suffix alkoxy to the name of the alkane i.e. Α , ω-position of the carbon chain are called polymethylene dihalides.All types of dihalides are called dihaloalkanes, the position of the halogen atoms being indicated by lowest possible arabic numerals.
Or R-CO-R’ where R and R’ may be same or different alkyl group.If R = R’ , ketones are called simple ketones.If R≠ R’ , ketones are called mixed ketones.Common name: In case of mixed ketones , name the alkyl group in alphabetical order and then add the word ketone.In simple ketones, the numerical prefix di is used before the name of the alkyl group.


 0 kommentar(er)
0 kommentar(er)
